portions adapted from Voet
& Voet Biochemistry 2nd edition
& Mathews & Van Holde
adapted for the web by L. Van Warren
Introduction:
Each of these sections are relatively brief and you are expected to read each section in its entirety. However section can stand alone as one or more lectures or even as a course. We have a single lecture and one interactive session.
Lecture Focus:
1 An overview of
metabolic pathways: What lies ahead in the course.
2. Some aspects of
bioenergetics & the concept of free energy.
3. The experimental
approaches used in metabolic studies.
Interactive Session (and Exam Question) Focus:
Solution of the problems at the end of the chapter. You will be expected to be able to explain to your classmates how you solved a problem when called upon Monday. You may omit problems 2, 9, & 13 for Monday's session but it is suggested you do them as part of your review for the Level 1 examination coming up in June.
This approach downplays the role an understanding of organic reaction mechanisms can play in learning metabolic pathways. Your text categorizes metabolic reactions into four types which reminds one that biological systems, either in vivo or in vitro, obey the same basic principles of chemical reactivity learned from studies of non-biological systems. A text written by Abeles, Frey and Jencks expands this view to where a student is encouraged to memorize a few concepts such as amino acid and nucleic acid base structures. Metabolic pathways are to be rationalized in terms of chemical principles. Experience has taught me that students who have a good grasp of organic reaction mechanisms can use this knowledge to their advantage in the study of metabolism. Students who do not have this understanding are unable to develop it based on 1 lecture. Certainly Dr. Raney presented some of these concepts in discussing enzyme catalysis.
I understand that Dr. Drake covered some aspects of the thermodynamics of phosphate compounds. Use the information presented in Chapt 15 to consolidate your knowledge in this regard.
Biochemistry is
the coupling of information and energy through catalysis. The
energy currency of the cell, ATP primarily, and how it is spent
is essential to understanding cell function.
The properties of some individual
enzymes and control mechanisms that affect their activity should have been considered.
The focus now will be on how a cell regulates its myriad of chemical reaction
sequences and how it controls its internal environment. Specific reaction pathways,
the relationship between each pathway and cellular architecture, the biological
importance of each pathway, the control mechanisms that regulate flux (flow
of material through each pathway) are all themes that will dominate the remainder
of this course and Biological Chemistry II. Within this mass of detail it is
important to keep the larger picture in focus.
See the whole forest
while looking at the trees.
Intellectual food is like any other; it is pleasanter and more beneficial to take it with a spoon than with a shovel. - Mark Twain
Metabolism is the totality of these consecutive linked reactions. Metabolism is a highly coordinated cellular activity in which many enzyme systems cooperate to accomplish:
1) Obtain chemical energy by capturing
solar energy or by degrading energy-rich nutrients from the environment.
2) Convert nutrient molecules into
the cell's own characteristic molecules.
3) Polymerize monomeric precursors
into macromolecules, proteins, nucleic acids etc.
4) Synthesis and degrade biomolecules
required in specialized cellular functions.
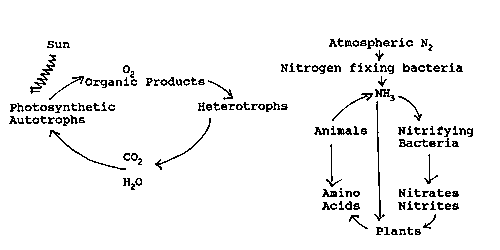
Living organisms can be divided into two groups:
1) Autotrophs which use C02 as their sole source of carbon.
2) Heterotrophs which cannot use C02 and must obtain carbon from their environment in the form of more complex organic molecules.
All living systems
require the elements carbon, oxygen, and nitrogen. Their interdependence
within the biosphere can be illustrated by these figures. Carbon,
oxygen, and nitrogen recycle continuously, but energy is constantly
transformed into unusable forms (heat and entropy).

Metabolism occurs in a series
of enzyme-catalyzed reactions that constitute metabolic pathways. In
a pathway a precursor molecule is converted into a product through a
series of molecular intermediates; metabolites. Metabolite means any
participant (substrate, intermediate, or product) in a metabolic reaction.
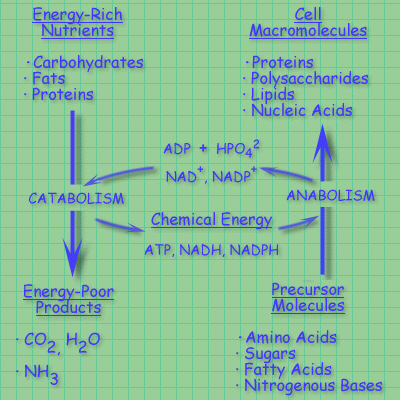
Catabolism is the degradative phase of metabolism, in which complex
nutrient molecules (proteins carbohydrates, fats) are converted to simpler end
products (lactate, CO2, NH3). Catabolic pathways release
DG, some of which is conserved in the formation
of ATP and reduced electron carriers (NADH & NADPH).
Anabolism (biosynthesis) is the conversion of simple to complex molecules.
Anabolic reactions require the input of DG,
generally in the form of the DG of hydrolysis
of ATP and the reducing power of NADH/NADPH.
Intermediary Metabolism: all reactions concerned with storing and generating
metabolic energy and use of that energy in the biosynthesis of low-molecular
weight compounds.
Energy Metabolism: pathways that store or generate metabolic energy.
Energy relationships between catabolic and anabolic pathways:
Catabolic pathways deliver chemical energy in the form of ATP, NADH, and NADPH.
These are used in anabolic pathways to convert small precursor molecules into
cellular macromolecules.
|
(c) deLuca/Warren |
Life in an Oxidizing Environment
Oxidation-reduction reactions involve the transfer of electrons between molecular species. Living things derive most of their free energy from them. Remember for each oxidative half reaction one must have a reductive half reaction.
Example: The most important reaction for our survival on this planet:
Reduction :
C02 + 2H+ + 2e- ---> (CH2O) (carbohydrate)
Oxidation :
H2O ---> 02 + 2H+ + 2e-
Summation :
C02 + H2O ---hv--> carbohydrate + 02
Photosynthesis
This endergonic reaction is driven by light energy. The fixation of approximately 1011 tons of carbon per year represents the storage of over 1018 kj (2 x 1017 kcal) of energy.
Aerobic organisms:
The predominate mechanism an aerobic organism uses to trap this energy is to couple the oxidation process to the synthesis of ATP.
Carbohydrate oxidation is NOT the reverse of the photosynthetic reaction. Reason:
Oxygen is a diradical species (triplet state) and,
as such, has to be reduced one (1) electron
at a time, not the two (2) electron process
written above. Carbon compounds usually undergo two (2) electron oxidation reactions.
Since oxidation/reduction reactions have to be coupled aerobic organisms a scheme(s)
is needed to allow this. Remember the role of flavins as either one or two electron
acceptors? These schemes are where the concept of reducing potential comes into
play.
Organisms like to have control of what they need to do to survive. Organisms
prefer a series of small changes in energy steps (DG)
for better control rather than one large energy change.
Therefore one would expect a biological system to
solve the problem of oxygen reduction via a series of coupled reactions involving
small energy changes per step. The sequence these reactions take is dictated
by thermodynamics as expressed in terms of reduction potentials.
Reduction potentials are a method of measuring reducing power.
Exergonic Nature of Most Substrate Oxidations
In aerobic organisms most of the needed energy release is derived from the oxidation of organic substances.
C6H1206 + 6 O2 ---> 6 CO2 + 6 H20 AG0 = - 2870 kj/mol.
Combustion of glucose in a calorimeter vs. complete metabolic oxidation of glucose differ in that the latter situation about 40% of the released 2870 kJ/mol is made available to drive the synthesis of ATP from ADP and Pi.
Relatively few metabolic reactions involve direct transfer of electron from a reduced substrate to oxygen. A series of oxidation-reduction reactions occur (electron transport chain or respiratory chain) with electron passed to intermediate electron carriers such as NAD+ and finally to 02, the terminal electron acceptor. This means that the potential energy stored in the organic substrate is released in small increments, facilitating control of the oxidation process and capture of some of the energy released. (Capture of transformed energy is what constitutes the extraction of energy into useful work, i.e. harnessing the "burning" of fuel. )
Energy Yields, Respiratory Quotient, and
Reducing Equivalents
If metabolic energy comes primarily from oxidative
reactions, it follows that the more highly reduced a substrate, the higher
its potential for generating biological energy.
Fat has a higher caloric content (-DG
released upon oxidation) than carbohydrate.
glucose C6H12O6 + 6 O2 ---> 6 CO2 + 6 H20 DG0 = -15.64 kj/g M.W. 180.16
palmitate C16H3206 + 23 02 --> 16 CO2 + 16 H20 DG0 = 38.90 kj/g M.W. 256.42
Respiratory Quotient (RQ) moles C02 produced/ moles 02 consumed
Glucose RQ = 6 C02/6 02 = 1
Palmitic Acid RQ = 16 C02/23 02 = 0.7
In general, the lower RQ the greater the
potential per mole of substrate for generating energy.
May also be expressed in terms of reducing equivalents.
A reducing equivalent can be defined roughly as 1 mol of H atom (one proton + one electron) Therefore two (2) reducing equivalents are used in the reduction of 1 oxygen.
1/2 02 + 2 e- + 2 H+ ---> H20
If catabolism involves primarily energy-yielding
oxidative pathways, it follows that biosynthesis of complex organic molecules
requires both energy and reducing equivalents.
8 carbon atoms (methyl groups) at an oxidation
state of -3 x 8 = -24. 8 carbon atoms (carboxyl groups) with oxidation state
of +3 x 8 = + 24 Net = 0
1 carbon atom (methyl group) oxidation state of
-3 x 1 = -3
14 carbon atoms (CH2) groups) oxidation
state of -2 x 14 = -28
1 carbon atom (carboxyl) oxidation state of +3 x 1 = +3 Net
= -28
Oxidation State: The actual or fictitious charge an atom would have if all the electrons of each bond were assigned exclusively to the more electronegative of the bonded atoms.
Need reducing equivalents to effect
the biological transformation:
Reductive biosynthesis: major electron source is NADPH
Generalization: Anabolism NADP+/NADPH Catabolism NAD+/NADH
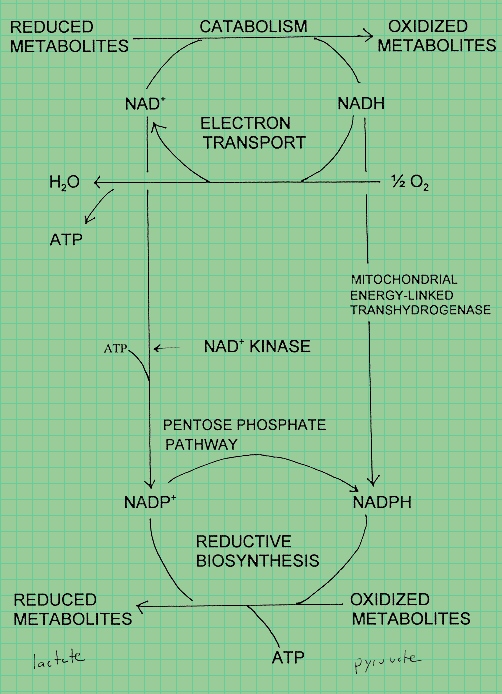 |
Oxidative electron flows in catabolism
and reductive electron flows in biosynthesis as seen in nicotinamide metabolism.
NAD+ the cofactor for most enzymes acting in the direction of substrate oxidation.
NADPH usually functions as the reductive cofactor.
Energy Transduction
Chemical energy stored in ATP can
be converted to other forms of energy, the process of energy transduction.
Examples:
Chemical to Mechanical Energy - muscle contraction
Chemical to Electrical Energy
- membrane depolarization
Why is ATP the "energy currency" of the cell?
1) ATP has a central position in the list of DG0' values for hydrolysis.
|
Phosphoenolpyruvate
(-14.8 kcal/mol) |
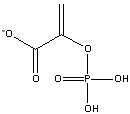 |
|
|
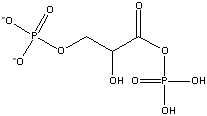
Glycerate-1,3-bisphosphate
(-11.8 kcal/mol) |
 |
|
|
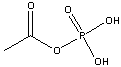
Acetyl Phosphate
(-11.3 kcal/mol) |
 |
|
|
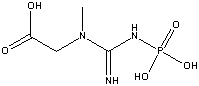
Phosphocreatine
(-10.3 kcal/mol) |
 |
|
|
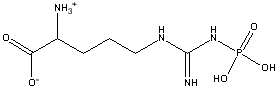
Phosphoarginine
(-9.1 kcal/mol) |
 |
|
|
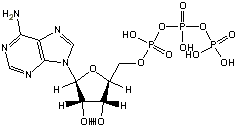
ATP
(-7.5 kcal/mol) |
 |
|
|
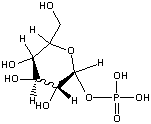
Glucose-1-phosphate
(-5.0 kcal/mol) |
 |
|
|
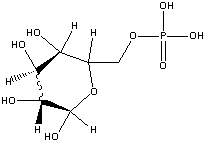
Glucose-6-phosphate
(-3.3 kcal/mol) |
 |
|
|
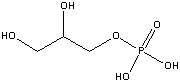
Glycerol-3-phosphate
(-2.2 kcal/mol) |
 |
|
|
Standard free energies of hydrolysis for some common phosphorylated compounds. The DG0' value refers to hydrolysis of the phosphate group. Given equal concentrations of the reactants and products, ADP can accept a phosphate from any of the compounds above it, and ATP can donate a phosphate to the unphosphorylated forms of the compounds below it. (c) deLuca/Warren |
||
2) How about other nucleoside triphosphates? Energetically they would seem to work as well.
3) The answer seems to be that Mother
Nature had to make a choice, picked ATP, and designed the rest of the system
around it. Intracellular concentrations of ATP range from 2- 5 mM, considerably
higher than other nucleoside triphosphates.
When other nucleoside triphosphates are needed,
they can be synthesized in a phosphate group transfer reaction catalyzed by
nucleoBide diphosphokinase.
How about compounds with higher DG0' of hydrolysis? Consider phosphocreatine in vertebrate skeletal muscle :
Creatine phosphokinase:
Creatine + ATP ------------> Phosphocreatine + ADP
Phosphocreatine + H20 = Creatine + Pi DG0' = -10.3 kcal/mol
ATP + H20 = ADP + Pi DG0' = - 7.5 kcal/mol
Phosphocreatine synthesis: DG0' = + 2.8 kcal/mol @ 37 deg. C K = 0.63 eq
The reaction is endergonic.
Phosphocreatine synthesis occurs in mitochondria where the [ATP) is high, thus the reaction is driven by Lie Chatelier's Principle.
Phosphocreatine diffuses from the mitochondria to the myofibrils, where high levels of ADP have resulted from contraction. Now the synthesis of ATP is favored.
Lesson: In predicting the direction of a reaction in vivo, consider both the energy change under standard conditions and the actual concentrations of all reactants and products in the cellular compartment where the reaction is carried out.
The central metabolic pathways are actually few in number and are remarkably similar for all forms of life. Metabolic pathways have four (4) principle (generally) characteristics:
1) Metabolic pathways are irreversible so that if two metabolites are interconvertible, the synthetic route from the first to the second must differ from the second to the first. Avoid futile cycles.
2) Every metabolic pathway has an exergonic first committed step.
3) All metabolic pathways are regulated, usually the first committed step.
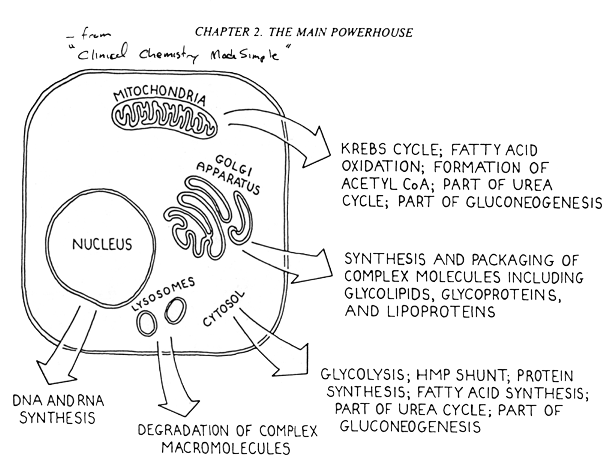
4) Metabolic pathways in eukaryotes occur in specific subaellular compartments. See attached figure.
Three types of nonlinear metabolic pathways:
a) converging, usually associated with catabolic pathways.
b) diverging, usually associated with anabolic pathways.
c) a cyclic pathway where one of the starting material, oxaloacetate, is regenerated and reenters the pathway. Acetate can be produced by the breakdown of a variety of fuels, can serve as the precursor for the biosynthesis of an array of products, or can be consumed in the catabolic pathway known as the citric acid (TCA) cycle. The TCA cycle is amphibolic it operates both catabolically and anabolically, as indicated below.
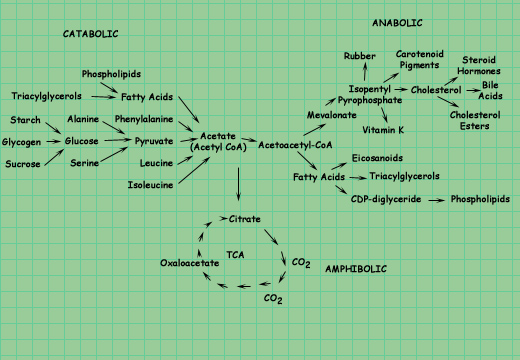
(c) deLuca, Warren |
Figure B The Three Stages of Catabolism: Based on structural complexity of the metabolites.
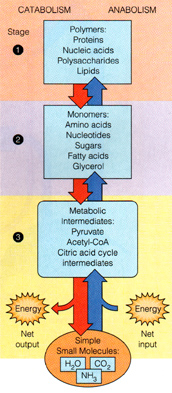 |
after Mathews et. al.
Stage 1: Reactions that interconvert
polymeric metabolites to their monomeric constituents.
Stage 2: These monomers further
degraded to simple intermediates.
Stage 3: Pathways that carry
out ultimate degradation to C02, H20, and NH3.
In aerobic organisms the principle stage 3 pathway is the citric acid
(TCA) cycle.
The Major Metabolic Pathways of Catabolism
1. Oxidative reactions of the TCA cycle drive ATP biosynthesis primarily through electron transport and oxidative phosphorylation
2. Glycolysis a Stage 2 pathway that catabolizes monosaccharides to pyruvate whose major fate in some tissues is oxidation in the TCA cycle.
3. Fatty acids in aerobic organisms are catabolized by p oxidation to yield the activated two carbon fragment acetyl COA. Acetyl-COA can be oxidized further in the TCA cycle or used to synthesize fatty acids and cholesterol.
Existence of Separate Biosynthetic and Degradative Pathways
Fatty acids are synthesized from Ac-COA & converted to Ac COA by p-oxidation. Pyruvate is a precursor of G-6-P via gluconeoqenesis but not by the reversal of glycolysis. Opposed pathways are distinct although may share common intermediates or enzymatic reactions, are regulated by distinct mechanisms and with different enzyme catalyzing their regulated reactions. Fatty acid synthesis is a cytosolic reaction whereas p-oxidation takes place in the mitochondria.
Why catabolic and anabolic pathways must be distinct:
1) A pathway must be exergonic overall to proceed. Simple reversal would be endergonic and thermodynamically unfavored.
2) Cellular need to control flow
of metabolites relative to the energy status of the cell. If the energy level
is high (high [ATP)) carbon can be stored so fatty acid oxidation, gluconeogenesis,
etc can come into play. If [ATP) is low and energy needed, stored carbon must
be mobilized to generate substrates for oxidation in the TCA cycle.
Separate
pathways crucial for control.
If fatty acid synthesis and degradation took place in the same cell
compartment and in an uncontrolled fashion.
A futile cycle, no useful work done, net result is consumption of the ATP required by the endergonic reactions of fatty acid biosynthesis.
The following example is a case where the two enzymatic activities occur on different domains of the same protein molecule. Dephosphorylation activates PFK-2.
 |
PFK catalyses a reaction of glycolysis (sugar catabolism) FBPase catalyses a reaction of gluconeogenesis (sugar anabolism)
Both enzymes respond to allosteric effectors, such that one is inhibited by conditions that activates the other.
Major Metabolic Control Mechanisms
Control of Enzyme Concentration
Levels of different enzymes vary widely. As expected, enzymes involved in the major metabolic pathways are present in greater amounts than those involved in some limited or specialized function. Known from two-dimensional gel electrophoresis.
Levels of a single protein can vary under different environmental conditions. Addition of substrate may increase the abundance of enzymes needed to utilize the substrate through synthesis of new proteins. Process termed enzyme induction. The presence of a pathway end-product may turn off protein synthesis, a process termed repression.
Control of Catalytic Activity
1) Interaction with ligands
a) Substrate concentrations
usually in the order of magnitude of Km.
Hence enzymatic activities are responsive to small changes
in [S].
b) Allosteric activators or
'inhibitors usually act on committed steps of a metabolic pathway, often
the initial reaction or branch points.
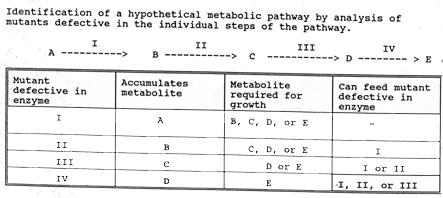
The feedback inhibition in the synthesis of the amino acids lysine, methionine,
threonine, and isoleucine in bacteria is illustrated in the following figure.
Note the positions at which products "feed back" to inhibit enzymes.
Also note that three different enzymes (isoenzymes) catalyze the initial reaction,
each inhibited by a different product.
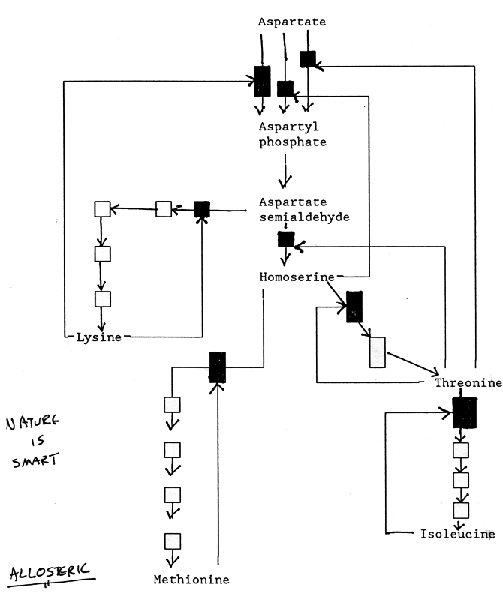 |
| Feedback inhibition in the synthesis of the amino acids lysine, methionine, threonine, and isoleucine in bacteria. The "black boxes" indicated positions at which products "feedback to inhibit enzymes. Note that three different enzymes (isozymes) catalyzed the initial reaction, each inhibited by a different product. |
2) Covalent modification of the
protein molecule
a) Phosphorylation, Adenylylation, Disulfide reduction, Proteolytic cleavage
b) Covalent modification often associated with a regulatory cascade.
Examples are glycogen breakdown, blood clotting, oncogenies
The concept of a regulatory cascade will be repeatedly encountered in your study of hormonal control of metabolism. Presumably you can build on this concept as illustrated by Dr.Drake with blood clotting.
Overview of metabolism, showing the central pathways and key intermediates. In general, catabolic pathways proceed downward (gray arrows) and anabolic pathways proceed upward (white arrows). Note that autotrophs have been grafted into this scheme.
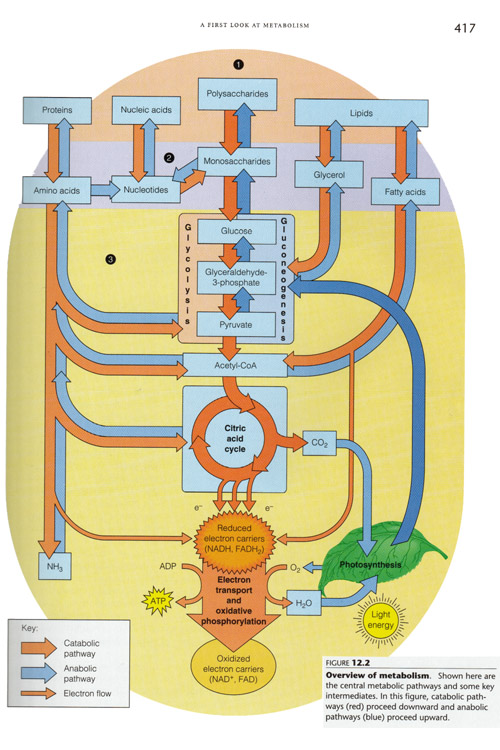 |
after Mathews et. al.
3) Compartmentation
a) Serves an important regulatory function by the selective permeability of membranes to different metabolites which controls passage of intermediates from one compartment into another. Specific carriers allow substrates to enter and products to exit. Flux through a pathway regulated by controlling these rates.
An example is insulin which activates (CH20) utilization by increasing the number of glucose transporters in the plasma membrane, so glucose is more readily taken into the cells for catabolism or for glycogen synthesis.
May be morphogenetically by the enzymes being bound to one another in a membrane - the enzymes of mitochondrial electron transport.
May be a highly organized multiprotein complex - the pyruvate dehydrogenase complex, a major entry point into the TCA cycle.
Hormonal Regulation
A hormone is a chemical messenger. It interacts with specific receptors, resulting in specific metabolic changes in the target cell. These changes are effected by all of the regulatory mechanisms cited so far: changes in enzymatic activity, changes in enzyme concentration, and changes in membrane permeability.
Insulin as an example: synthesized in the pancreas and affects target cells in liver, muscle, and adipose tissue, acts a all three levels to promote anabolic pathways and inhibit catabolism.
Goals of Experimental Analysis of Metabolism
For a particular metabolic process:
1) identify reactants, products,
and cofactors, plus the stoichiometry, for each reaction involved.
2) understand how the rate of
each reaction is controlled in the tissue of origin.
3) identify the physiological
function of each reaction and control mechanism.
Extrapolation from in vitro to in vivo is challenging and important. For example:
The mitochondrial enzyme that synthesizes ATP from ADP was initially isolated as an ATPase (catalyzed the hydrolysis of ATP to ADP). Must show that the same enzyme is catalyzing the same reaction in intact tissue.
Levels of Organization at which Ketabolism is Studied
1) Whole Organism (Will illustrate
for mammalian species)
2) Isolated or Perfused Organ
3) Whole Cells - Slices or Cultured
Cells
4) Cell-Free Systems - Homogenates
5) Purified Components
Fundamental "Probes" of Metabolism
2) Reaction Blockade
a) Metabolic Inhibitors
b) Genetic Alterations
A. Whole Animal Studies
1) Nutritional requirements for normal growth and
development:
This sets the boundary conditions!
a) Essential elements (minerals)
b) Essential amino acids: Concept of Nitrogen Balance
Nitrogen balance = Nitrogen intake - (Urinary Nitrogen + Fecal output)
c) Essential fatty acids: i.e linoleic, arachidonic, Q-linolenic
d) Vitamins:
The tools of the trade at this level are growth
rate, nitrogen balance determinations, pathology associated with deficiency
symptoms.
Essential Human Nutrients:
|
Essential Human Nutrients
= Water +
|
||
|
Amino
Acids
|
Elements
|
Vitamins &
Like Compounds
|
|
Isoleucine
Leucine Lysine Methionine Phenylalanine Threonine Tryptophan Valine |
Calcium
Chlorine Choline Copper Folic acid Iodine Niacin Iron Pyridoxine (B6) Magnesium Riboflavin (B2) Phosphorus Vitamin B12 Manganese Vitamins A, D, E, K Potassium Thiamine (B1) Sodium Biotin Chromium Pantothenic Acid |
Ascorbic acid |
|
Probable Essentials
|
||
|
Arginine |
Arsenic |
|
2) Experimentally Induced Alterations in the Organism
a) Surgical removal
of organs
Liver: role in
ammonia and urea metabolism
Pancreas: role in carbohydrate metabolism
b) Surgical access to the internal milieu: fistulae and cannulation etc.
Phlorizin - muscle phosphorylase a inhibitor interferes with reabsorption of glucose by the renal tubules
Alloxan - destroys p-cells of the pancreas - diabetes In animals so treated, certain amino acids cause the appearance of extra glucose in the urine whereas other amino acids induce the excretion of ketone bodies (acetoacetate, acetone, b-hydroxybutyrate). Glucogenic vs ketogenic amino acids.
3) Fate of molecules in the intact organism
a) Changes in body chemistry induced by feeding certain compounds. Assessment of changes in body tissue fluids and gases.
b) Following the fate of chemically tagged molecules - Knoop 1904 Omega phenyl- substituted straight chain carboxylic acids: even numbered excreted as phenylacetic acid (phenylacetylglycine derivative); odd numbered excreted as benzoic acid (benzoylglycine derivative). Therefore fatty acid oxidation occurs with the successive removal of 2-carbon units after the oxidation of the P-methylene to a b-keto group.
c) Following the fate of isotopically labeled materials; the concept of turnover studies.
i) Stable isotopes: H2 (D), N15, C13, and 018: Monitor by mass spectrometry.
ii) Radioisotopes: H3, C14, P32, S35, Ca45, 1131, 1125: Monitor radioactive decay process.
4) Genetic alterations of the organism
b) Mutant microorganisms: See the text, page 318. This should be integrated with the Genetic Biochemistry material.
5) Applications and limitations of whole organism experimentation
Many processes are occurring simultaneously hence there are many variables the investigator needs to control. The organism is still under hormonal and nervous control. The observed effects are difficult to establish as a direct series. Many indirect routes exist. From an experimentalist's viewpoint it may be impossible to have only one variable at a time. But, all results must eventually be compatible with whole animal studies. This doesn't say that in vitro results must also alvays be obtained in vivo. In most cases this is probably impossible.
B. Perfused organ studies
Many of the whole organism procedures are appl{cable here. The system is removed from hormonal and central nervous system (CNS) control and influences of other tissues. The cells are still intact.
C. Tissue slice studies
Used with soft tissues such as liver, kidney, and brain. Such tissues may be sliced approximately 50 microns thick. This allow enough surface area for exchange of nutrients and wastes so that cellular viability is maintained for several hours. Most of the cellular constituents remain in the cells. The technique allows control organ, preincubation treatment, nutritional status control, variability of the bathing fluid (incubation medium) and the gas phase. The technique has been especially useful in the study of respiration.
D.Tissue culture
The ability to grow cells in culture is tending to supplant the perfusion and slice techniques. These studies viewed as whole cell experiments that facilitate bridging the gap between studies using homogenates and whole organs.
E. Tissue homogenates
The classical biochemist's "grind and find" technique. Mechanical grinding of tissue results in the rupture of cellular membranes and destroys the relationship between various suborganelles of the cell. But it does facilitate the use of specific metabolic inhibitors to study metabolism. One reason for this is that the inhibitor does not have to permeate the cellular membrane, a process that may or may not be easy.
F. Subcellular partiales (fractions)
The technique involves isolation from homogenates by differential centrifugation thus is more operational than anatomical. Some applications are indicated in the following table and it is assumed this supplements Dr Bhuvan's material:
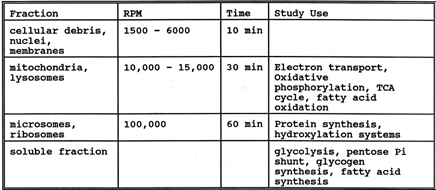 |
G. Purified enzymes
The step required for complete understanding of the individual reaction. The chemical action of purified enzymes is essential for understanding the role of the individual enzymes in the chemical dynamics of a biological unit.
Types of Radioisotope Tracer Experiments
1) Equilibrium labeling
2) Pulse labeling
3)
Pulse-chase labeling
Equilibrium labeling: Administer radiolabeled precursor and determine the location of the labeled atoms in the product. Exposure to the tracer is relatively long, so each labeled species reaches a constant specific activity (dpm/mol)
Bloch: 14C acetate -->-->-->--> 14C-cholesterol
Acetate administered to rats. Isolate cholesterol from the liver and determine, by chemical degradation, the specific carbon atoms in cholesterol that had incorporated radioactivity.
Pulse labeling: administration of an isotopic precursor for an interval that is short relative to the process under study. Radiolabeled material accumulates preferentially in the shortest- lived species, the earliest intermediates in a metabolic pathway.
Calvin Exposure of green algae to 14C0 as a function of time (seconds) to determine the patAway of photosynthetic carbon fixation. Ten second exposure - 15 compounds labeled. Five second exposure - 1 compound labeled - 3- phosphoglycerate
Pulse-chase labeling: administer label for a short period of time and rapidly reduce the specific activity of the isotopic precursor to prevent further incorporation. Samples are assayed as a function of time after the pulse to determine the metabolic fate of the material labeled during the pulse. This was how mRNA was originally detected.
Precursor - Product Relationships - Zilversmit
Metabolic pathways normally operate in a steady
state where the flux of metabolites is constant. Flow (flux) is controlled by
the rate-limiting step. Most metabolic reactions are operating under first order
reactions conditions for a given substrate, substrate concentration is in the
region of or less.
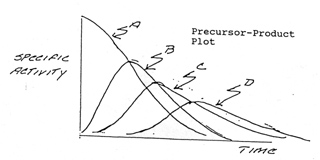
For the reaction sequence: A ---> B ---> C ---> D A pulse of radioactive
A (precursor) will show the following plot of specific radioactivity vs time
after addition of A for the products. A precursor will cross the apex of its
product curve.
 |
 |
 |
HOUSEKEEPING (GENTRALIZED) ORGAN FUNCTIONS
|
Organ localization
of some key biochemical reactions
|
|
|
SPECIALIZED
ORGAN FUNCTIONS
|
MAJOR
SITES
|
|
Fatty acid synthesis liver
Amino acid synthesis and breakdown liver |
liver, fat cells liver |
Problem:
Write a balanced equation for the reduction of molecular oxygen by reduced cytochrome c as carried out by complex IV (cytochrome oxidase) of the electron transport chain.
a. What is the standard free energy
change (DG0') for this reaction
if
DE0' cyt c(Fe3+)/cyt
c(Fe +) = + 0.254 volts and DE0'
(l/202/H20) = 0.816 volts?
b. What is the equilibrium constant (Keq) for this reaction?
c. Assume that (1) the actual free energy release accompanying cytochrome c oxidation by the electron transport pathway is equal to the amount released under standard conditions (as calculated in section a.), (2) this energy can be converted into the synthesis of ATP with an efficiency of 0.6 (that is, 60% of the energy released upon cytochrome c oxidation is captured in ATP synthesis, and (3) the reduction of 1 molecule of O2 by reduced cytochrome c leads to the phosphorylation of 2 equivalents of ATP.
Under these conditions, what is the maximum ratio of [ATP]/[ADP] attainable by oxidative phosphorylation with [Pi] = 3 mM? (Assume DG0' for ATP synthesis = + 30.5 kj/mol.)
Last Revised: Oct 27, 1999 LVW