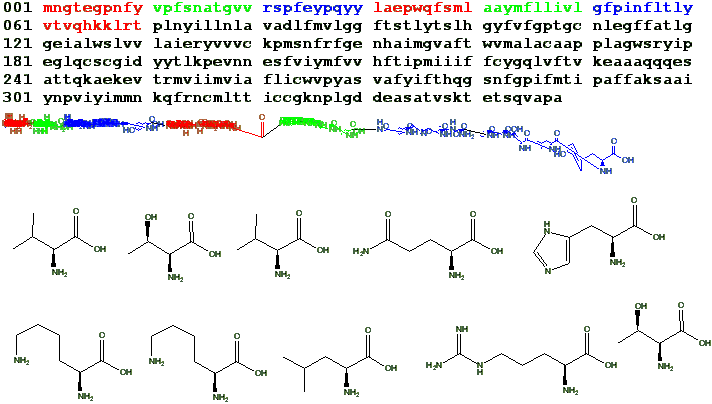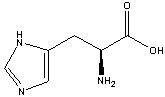
Question on Condensation:
Short Version:
How do I know that the peptide bonds I am choosing are actual orientations in
all cases?
Long version:
Take
histadine for example:

If we consider the aromatic hydrogen to be interchangeable with the double bond. The ring has two possible binding sites. Can't the hydrogen oscillate between two quantum configurations with relatively low energy penalty? Like so:
 VERSUS
VERSUS

Two configurations within a close delta energy on the ring implies two different proteins. So for the case of GH, glutamine-histadine we could have:
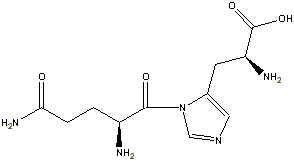 OR
OR 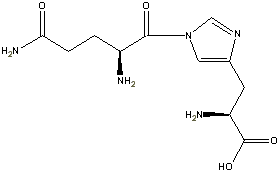
There is also another -NH2 closer to the carboxyl group, not bound into the ring at all. Why not that one? After all there are two hydrogens for dangling for all to see.
So what do I need?
I need a list of rules with underlying rationale for why specific N-H sites win OR
I need a list of the binding energies associated with each N-H site on each amino acid OR
Given that enzymatic (potential energy tampering) processes are at work, some kind of a sit down story on the synthesis mechanism that enforces only the endmost site on each amino acid. Then I could have some confidence in what is really quite a long molecular model and a lot of work, see below: