Introns
as Timers:
V. Warren November 5, 2005 |
|||||
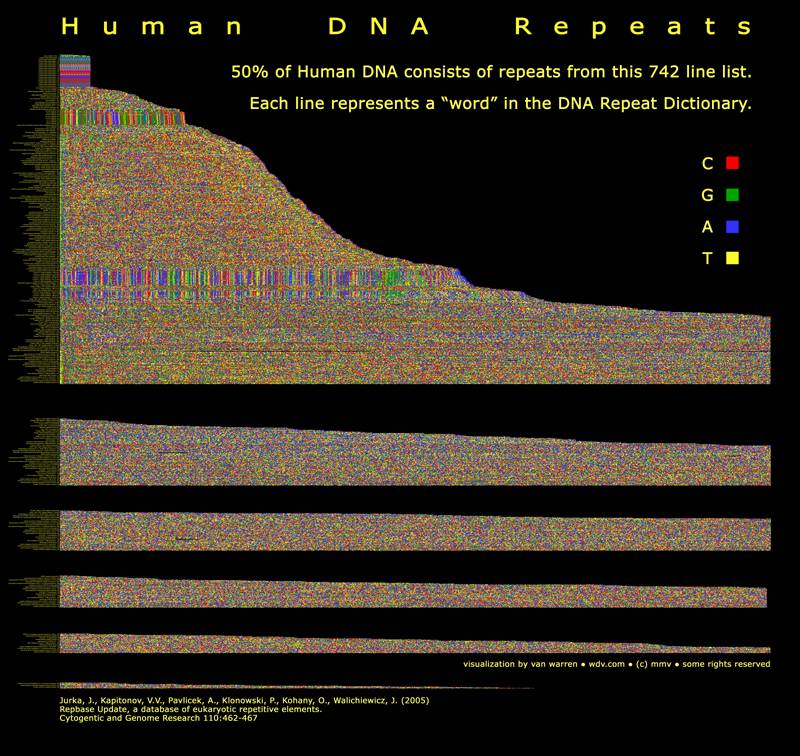
Introduction/HypothesesIt is often said that 96% of our DNA consists of spacers called introns that serve no useful purpose. The remaining DNA codes for proteins of metabolism and body plan. What if intronic DNA controls the rate of gene expression? Is intronic DNA is used for timing and sequencing purposes? Intronic DNA is classified in the mural below. The regions include microsatellites, SINES, LINES, ALU repeats and transposable DNA introduced by retrotransposons. Retrotransposons are also known as jumping genes. You can see the complete lexicon of repeats in the figure below. Fifty percent of our DNA is drawn from this list of repeats. That is interesting donít you think? Successive clicks on the mural below lead to larger versions of the mural. |
|||||
At risk of repeat: Does intron length and frequency is influence the rate of transcription and if so, to what degree? Is the function of non-coding DNA related to timing or temporal regulation?
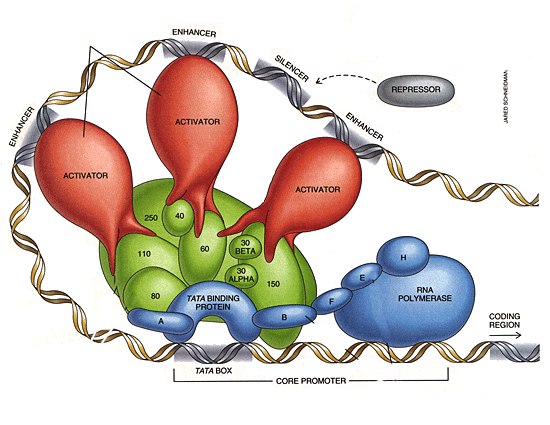
Rationale:In higher eukaryotes, introns are more frequent and longer. They are particularly long in genes which are associated with high complexity, like factors in the transcription apparatus. As complexity increases, so does the need for things to be done "in sequence", at the right time, and in the right way. This includes assembly and temporal regulation of RNA Polymerase II and its cofactors. |
|||||
-- after Tijien et. al. sciam.com |
|||||
For example, if the ALU repeats that populate the BRCA1 gene are there to control the rate at which the transcript is produced, upstream intron length affects downstream translation rates i.e. the "time to assemble" the transcription apparatus.
Consider the following diagram of the BRCA1 transcription factor gene. The recognizable ALU repeats are distributed throughout the gene. We can sort the introns and exons to produce a more coherent diagram that shows insertion, deletion, and substitution mutations remarkably well. |
|||||
|
|||||
The coding part of this gene is only the part above the ALU repeats and microsatellites.
If true transposable elements including retrovirus fragments and other cosmic litter confer an advantage on the organism when populating the DNA, improvements in timing confer a survival advantage. In fact, one could look on timing optimizations as gain of function mutations.
The point is this Ė if intronic DNA serves a timing or rate control purpose, then retrotransposition should be recognized as another mechanism of organism adaptation.
This would also hold in morphogenetic sequences where layout of body plan is very time sensitive. Operations carried out at the wrong rate could result in failed body plans. Further examination of the HOX master control body planning gene clusters would provide additional insight. Consider an obvious analogy: If one's hand developed to completion before the neural or circulatory support was available, the result could be catastrophic.
Therefore, my hypothesis is that junk DNA is used to control the timing of transcription and possibly downstream translation.
Presume that the temporal availability of RNA transcripts is affected by frequency and placement of introns. Consider factors which affect the rate of RNA self-annealing. Do the formation of stem and loop structures influence the output rate of the transcript or vica versa Shape and charge are important. So is availability. ConclusionAn experiment to assess this hypothesis would change the lengths of introns and see if it interferes with body plan, development, maturation, metabolism, and adaptation on different time scales. Could timing considerations provide insight as to why repeat diseases like Huntington's chorea grow worse in later life? A therapeutic benefit; Timing could be modified by insertion or deletion of introns. Those are my questions for now. |
|||||
†††††††††††††††††††††††††††††††††††††††††† ††