An Adventure in ChemOffice™
and ChemINDEX™
Courtesy CambridgeSoft and Eldest Daughter
L. Van Warren
Today’s
review is of CambridgeSoft™ ChemINDEX™, by way of a
short saga that out of necessity involved much of the ChemOffice™
toolkit. This illustrated adventure has some unexpected twists and turns, and a
surprise conclusion.
Background:
I have been a long time user of Cambridge Software’s ChemFinder™ product. ChemFinder™
is one of the great internet science institutions. Name any chemical you can
think of, and everything you want to know about it is at your fingertips,
courtesy our
My
daughter came home from her high school chemistry class with a seemingly simple
problem. Create a simple toothpick and foam ball model of
2-bromo-3-methylpropanal. Sounds like that college course, IUPAC 101, which by
ancient foggy recipe goes something like:
- Make a propane
- Double bond
an oxygen to carbon one. This makes propane go propanal.
- Stick a bromine on carbon two
- Add a methyl on carbon three.
What could be easier?
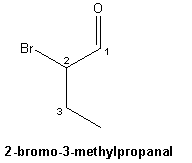
Well a lot
of things probably, but this was the night of the final, and my
daughter, showing her usual modicum of due diligence, had also brought the
structure home after confirming with the teacher that it was correct. With unwavering
faith in the infallibility of her teacher, all that was left
was to stick some foam on some toothpicks. There starts the trouble. The
approved structure counted for the last and most important 100 points of the
semester. The structure and formula were sketched on a
napkin in blue ball pen ink. You know the story, “as the goes the final, so
goes the class, the semester, the year, the college and career. The sketch of
this high stakes game of fate looked something like this:
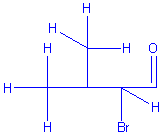
As a general rule, I stay out of my daughter’s assignments, she is quite low maintenance
and only asks question on rare occasions, which is frustrating. I’m happy to build her any size of clock when she asks for
the time. But something about this molecule bothered
me. I stayed quiet as the foam was painted in non-CPK
colors. I even endured temporarily as the model was
assembled completely on the flat plane of the table… with carbons lined up like
soldiers. But how much can a person take? The errors
were piling up. It was time for action, time to do or die, time to speak up for
tetradral bonding, to come out of the plane, to break
the surly bonds, or at least point them in the right direction! But the greatest teachers, “teach without words”. What to do?
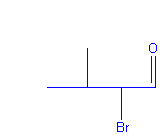
I pointed at the napkin and looked carefully at the name, then at the structure. I started quietly deducting hydrogen’s and thinking bad thoughts like, “Isn’t this a bad drawing of 2-bromo-3-methylbutanal?”
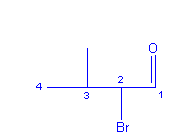
If I would have listened more carefully, I would have heard the
distinctive and quiet click that a land mine makes when you step on it. It
turns out that to even think of questioning the
chemistry teacher is like trying to fool mother nature. It can be done, but its usually not a good idea. My
daughter tersely informed me, “…propanal”. But I couldn’t get that fourth carbon out of my mind. I kept
counting them over and over, perhaps it was the
suffix, perhaps just me. Either way, whether you take the fork in the road or
not, that is a butane backbone girlfriend. My elderly
blathering interrupted the otherwise pristine concentration of my daughter who
was waiting for the white glue to dry. But in the
pause I could appeal to a higher authority! I flipped on my
computer, broke out the ChemDraw™ and drew the structure with the primordial
wiggles that got carbon going in the first place:
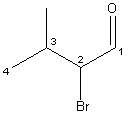
Perhaps
this was a “teachable moment”. Perhaps a little tiny tweak of a drawing would
open a door of insight never before imagined! Not only could I
prove the name was wrong, I could show that carbon structures don’t spend a lot
of time in the plane. I quietly went to the
name-that-structure feature in ChemDraw™. Convert structure to name I think it was. Instantly I had a
labeled structure:
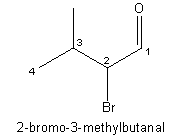
More importantly I had the proof from a higher (and also
automatic) authority that my IUPAC piety was indeed deserved, at least in this
rare instance. I called my daughter over and showed
her the computer applying the name. “Too bad your computer is
wrong too”, the denial came. Stunned I reflected, “It must be that my 2D diagram, even with its
tetrahedron reminiscent wiggles, is insufficient. I am
just perpetuating the myth of the plane myself! Now we have to get the big guns
out. Time for some 3D rendering, time for energy minimization and partial
charge, time for some real molecular modeling.” I tell
my daughter, “Yeah, this is flat picture, on a flat screen, let’s take it up a
level”. I save the structure and whip out Chem3D™
Ultra. Time to build a real clock now. That’s easy enough, just read the cdx file:
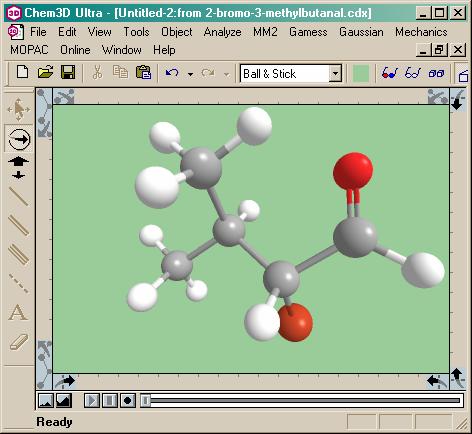
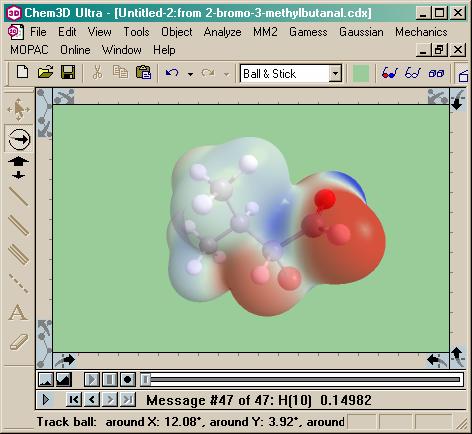
Now I’m rubbing my hands together. For a warm-up, we need to let
this molecule relax into its natural confirmation, complete with steric
hindrances. No problem. A little “Minimize Energy”
option off the MM2 menu does the trick with lightning speed. The only change? A
little double bonded oxygen, leaning back, waiting to
catch some rays. I wonder what photon would ring that
bond…
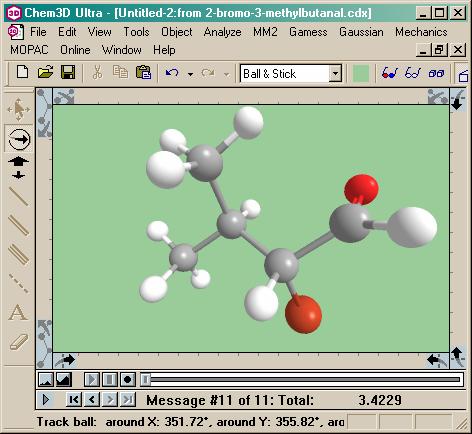
The light
is starting to shine, my daughter paces uneasily. This
could be a problem for her and the teacher and
Now we’re
cooking with heat. Then suddenly it happens. The lament begins. My daughter looks at her foam and toothpicks and exclaims,
“My teacher is wrong… I can’t believe my teacher is wrong, she has never been
wrong about anything ever”. “Hmm, all
this effort to prune by one carbon”, I think to
myself. So the teacher missed one carbon among fifty
students… that I could do so well. It’s just the process. “So let’s prune by
one carbon, verify the name and you can be done. You can turn in your flat
model, which is what the teacher wants, and you can also
get the chemistry right. All’s fair in learning and exploration.”
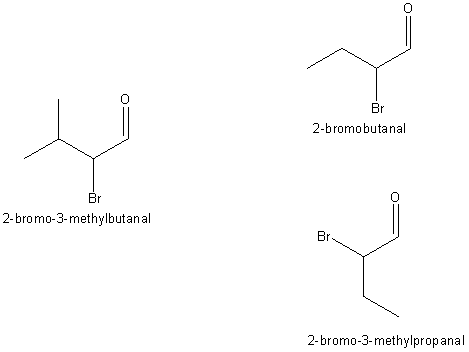
With
automation in hand we go back and prune by a carbon.
We run the names for all the cases. Suddenly an isomeric name pops up, like so,
completely unexpectedly, polluting the clear waters of unambiguous names:
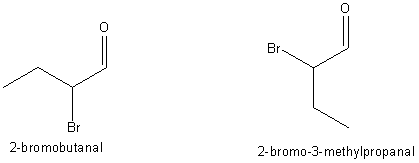
Just for
grins, we look at it in 3D, to see if the different name is really a different
chemical or just a “name isomer”. After all some simple molecules can possess
chiral, or handedness. They could be left or right handed versions of each
other, like thalidomide that destroys lives, and saves them by cutting off the
blood supply in certain cancers.. One racemate prevents infant limb development, the other doesn’t,
but that is all an aside. Watching our isomers wiggle courtesy of Chem3D
molecular dynamics shows that adjacent groups are free to spin on those single
bonds without lockdown from steric hindrance, so our two name-isomers are indeed
the same substance. We are almost done.
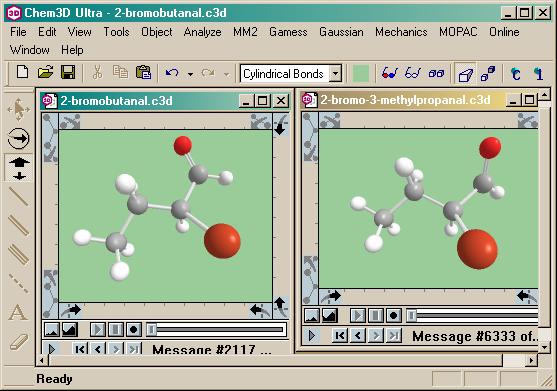
To wrap up
would like to know what the differences are between the organic compounds that
appeared in our exploration. Is one safe and the other toxic? Do they have
common uses? Do they smell bad… or good? One last visit to ChemFinder™
and we should be done. We are now comparing the first and last molecule, the
one the teacher drew and the one the teacher named.
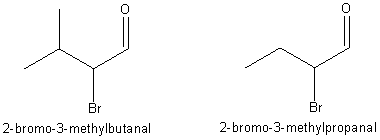
We jump to
our old friend Chemfinder.com and type in the names. Neither one of these show
up. Could these be rare and exotic compounds? Are they in any web based literature? This is no time to
have to trudge down to the library and wade through the chemical abstracts,
it is past
Van and eldest daughter reside at a
secret lab in the

van at wdv.com